For the first time, researchers have shown that alterations to two cell ‘pathways’ directly cause lung adenocarcinomas which could provide a breakthrough for its diagnosis and treatment
Lung cancer is Australia’s biggest cancer killer, and is expected to claim more than 9000 lives over the course of just this year.
Although we can reduce our risk of getting lung cancer by quitting smoking – or better still, never starting – this deadly disease cruelly catches many who have never had a single puff of a cigarette.
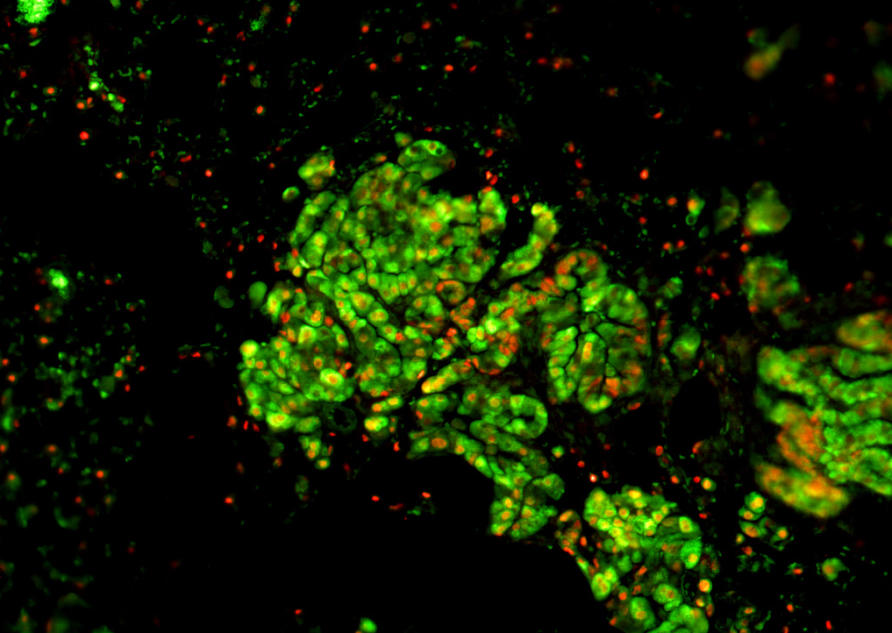
And the most common lung cancers affecting non-smokers are an aggressive type, called adenocarcinomas.
They account for around 40 per cent of all lung cancers and occur more frequently in females and in young people than other types of lung cancer.
Now, research from the Walter and Elizabeth Hall Medical Research Institute, University of Melbourne and the Bio21 Institute, published in Cell Metabolism, could lead to a new diagnostic blood test and treatment for adenocarcinoma.
Cancer researchers Dr Sarah Best and Dr Kate Sutherland focused their attention on alterations in two cell programs, or “pathways”, that instruct our cells how to behave.
Dr Best and Dr Sutherland already knew from previous studies that alterations in the KEAP1/NRF2 pathway had been identified in 23 per cent of lung adenocarcinomas, suggesting that deregulation of the pathway is a major cancer driver. This pathway normally functions to reduce toxins that lead to cell stress and shut-down, so activation of this program ensures cells can continue to function and grow.
The PI3K pathway alterations are found in 11 per cent of adenocarcinomas. This pathway drives cell growth and division, so it is important that regulation of this program is under control.
“Lung cells with both of these alterations grow faster and with less stress,” Dr Best says.
And when it comes to cancer, that is the last thing you want.

Using preclinical models, the researchers confirmed that lung adenocarcinomas are caused by mutations that lead to non-stop activation in both the KEAP1/NRF2 and PI3K pathways.
“This is the first time anyone has shown that these alterations directly cause lung adenocarcinomas.
“With this knowledge, we can further investigate how targeting the activity of those pathways could lead to therapies for these aggressive and hard-to-treat cancers,” Dr Best says.
But they also made two other important findings.
“The first is that these adenocarcinomas have characteristics that make them sensitive to immunotherapies, that are already being used to treat other types of cancer like melanoma,” she says.
This is significant because these tumours are chemotherapy and radiotherapy resistant, meaning there are effectively no current treatments available for these patients.
“Using preclinical models, we showed for the first time that these tumours have the ‘markers’ that make them susceptible to anti-PD-1 and anti-CTLA-4 immunotherapies, which are some of the most exciting new cancer therapies being investigated in the clinic.
“But more importantly, we showed that these immunotherapies were effective in fighting the tumours and leading to tumour regression in our preclinical models,” she says.
Normally, our immune system can detect and kill cancerous cells, so when a cancer develops it has evaded our immune system. Immunotherapy (such as anti-PD-1 and anti-CTLA-4) is an exciting new treatment option that can unmask the cancer to our immune system, unleashing its natural killing capacity.
Currently, the efficacy of immunotherapy to treat lung cancer is being investigated in clinical trials in Australia.
Their other finding is that the cell pathway changes create a distinctive metabolic by-product, such as an altered abundance of specific sugars, which can effectively be used to detect these hard-to-treat lung cancers.
Dr Best says all cells leave by-products in the blood, but adenocarcinomas with these alterations have their own fingerprint.
By collaborating with Bio21 Institute researchers Dr David De Souza and Professor Malcolm McConville in using mass spectrometry, the group was able to identify the unique molecular signature left by the adenocarcinoma.
As Dr Best says, identifying these distinctive cell by-products among all the other normal by-products is a bit like finding a jellybean in a bag full of gummy bears, resulting in a breadcrumb trail that leads straight to the lung.
But now that they’ve uncovered how to identify these “breadcrumbs” they believe that this knowledge could in future be used to develop a simple non-invasive blood test for these aggressive lung cancers.
And these unique molecular signatures found in the blood could soon be used as a tool to identify patients who would respond to those immunotherapies that Dr Best and Dr Sutherland have shown to be effective and which are already available.
“The next steps would be to analyse human samples to prove the same is true in lung adenocarcinoma patients,” Dr Sutherland says.
“But we need more funding for that work to continue and to generate results that will lead to better detection and treatments for the community.”
“This article was first published on Pursuit. Read the original article.”





















Add Comment